Periodic Safety Update Report (PSUR)

Dr Klinisch research provides support in preparing high quality Periodic Safety Update Report to all our clients.
Periodic Safety Update Report is prepared as per EU Medical Device Regulation 2017/745 of Annex III requirements.
A Periodic Safety Update Report summarizes the results and conclusion of the analysis of the Post-Market Surveillance data gathered as a result of the Post-Market Surveillance Plan.
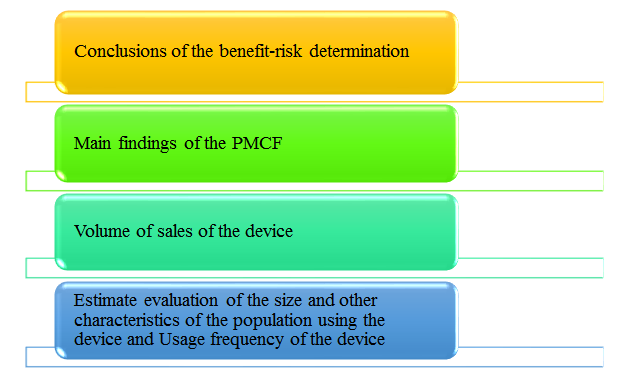
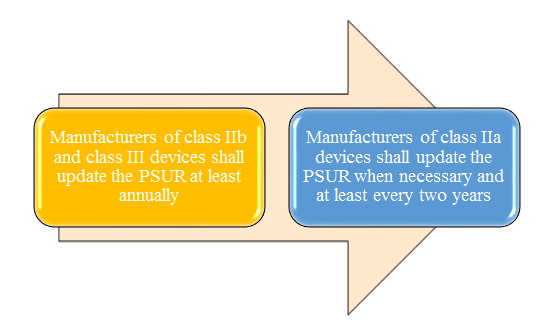
Manufacturers of class IIa, class IIb and class III devices shall prepare a periodic safety update report (‘PSUR’) for each device and where relevant for each category or group of devices summarising the results and conclusions of the analyses of the post-market surveillance data gathered as a result of the post-market surveillance plan with a rationale and description of any preventive and corrective actions taken. Throughout the lifetime of the device concerned, that PSUR shall set out:
When to update your device PSUR
For class III devices or implantable devices, manufacturers shall submit PSURs by means of the electronic system referred to in Article 92 of MDR to the notified body involved in the conformity assessment in accordance with Article 52 of MDR. The notified body shall review the report and add its evaluation to that electronic system with details of any action taken. Such PSURs and the evaluation by the notified body shall be made available to competent authorities through that electronic system.
For devices other than class III devices or implantable devices, manufacturers shall make PSURs available to the notified body involved in the conformity assessment and, upon request, to competent authorities.
